Comprehensive Guide to Polyacrylic Acid and Sodium Polyacrylate
Understanding Polyacrylic Acid and Sodium Polyacrylate: Basics and How to Choose
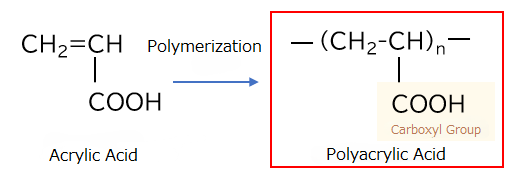
What is Polyacrylic Acid?
Polyacrylic acid is a water-soluble polymer obtained by polymerizing acrylic acid. It is a type of polycarboxylic acid that contains carboxyl groups. At Nippon Shokubai, we have maintained a vertically integrated production system from acrylic acid since the 1970s, ensuring a stable and reliable supply of polyacrylic acids.
Polyacrylic acids with a broad spectrum of molecular weights have been developed. Due to their molecular weight-dependent characteristics, they are utilized in a wide variety of applications.
What is Sodium Polyacrylate?
Neutralizing polyacrylic acid with sodium hydroxide produces sodium polyacrylate by converting its carboxyl groups into sodium salts. Sodium polyacrylate has different properties from polyacrylic acid. For example, aqueous solutions of high molecular weight sodium polyacrylate are often used as thickeners.
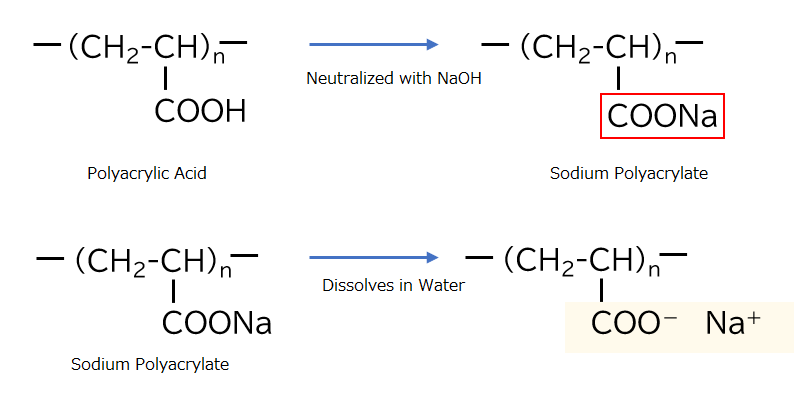
The Difference between Polyacrylic Acid and Sodium Polyacrylate
Polyacrylic acid contains multiple carboxyl groups in its molecular structure but does not exhibit internal electrostatic repulsion. In contrast, the neutralized form—sodium polyacrylate—experiences electrostatic repulsion within the molecule, which causes the molecular chains to expand more easily.
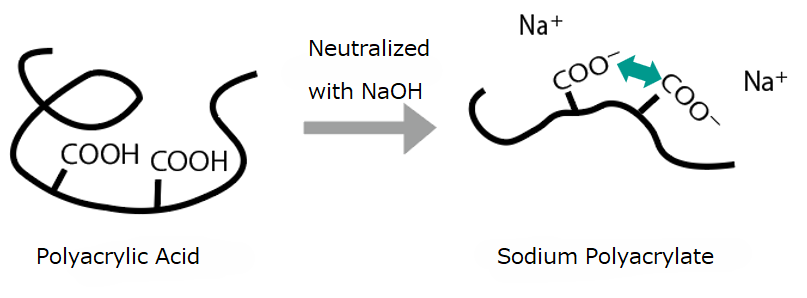
As shown above, due to the expanded molecular chains, sodium polyacrylate exhibits a stronger thickening effect.
How to Choose from a Variety of Polyacrylic Acids and Sodium Polyacrylates
By Application: Based on Molecular Weight
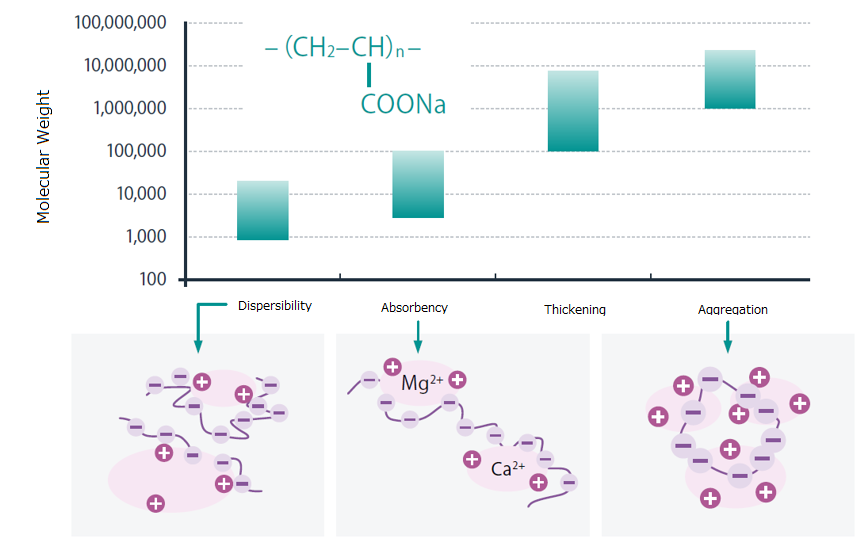
Polyacrylic acid and sodium polyacrylate exhibit different functionalities depending on their molecular weight. The following is a general guideline for typical applications. While the molecular weights listed indicate those that tend to deliver high performance, effective functionality may also be observed at other molecular weights.
■Dispersant ― Mw: 1,000 – 10,000 (Mw: Molecular weight)
Adsorbs onto solid surfaces and uses electrostatic repulsion between solids to stabilize slurries or disperse various components.
■Chelating agent ― Mw: Several thousand – around 100,000
Captures metal ions (e.g., calcium ions) and is used in detergents as a chelating agent. It is safer than low molecular weight chelating agents.
■ Thickener ― Mw: 100,000 – several million
High molecular weight types exhibit thickening and stringiness. Sodium polyacrylate, due to internal electrostatic repulsion, shows even stronger thickening effects. Viscosity can be adjusted by changing concentration and pH
■Flocculant ― Mw: >1,000,000
High molecular weight polyacrylic acid (or sodium salt) bridges particles, providing strong flocculation. Used in wastewater treatment and impurity removal in food production.
By Physical Form
There are powder and aqueous solution types. If both are available, the aqueous solution type is chosen to save time on dissolution, while the powder type is selected when water addition is undesirable.
By pH
Polyacrylic acid in aqueous solution is acidic (pH ~4). Neutralized sodium polyacrylate is neutral to alkaline. As a thickener, it shows the highest thickening effect around pH 10, resulting in high viscosity.