In development Graphene Oxide
- Main Applications
Scaffolding materials (Primers), Heat-conductivity materials, Antibacterial and antiviral materials, Electronic information materials, Transparent conductive films, Water treatment membranes, Sporting goods, Battery materials, Conductive ink, Lubricants, Aerospace materials, Biosensor materials, Millimeter-wave absorbing materials, etc.
- Applications
- Functions
Basic Information
What is Graphene Oxide?
Graphene oxide is a type of nanocarbon material. It has a high aspect ratio and high surface area, with a 1 nm thickness and a sheet length of a few to several dozen microns.
Graphene oxide has a sheet structure with rich oxygen-functional groups. It also has high dispersibility, which so far has been difficult to achieve in existing nanocarbon materials such as carbon nanotubes and graphene. This enables graphene oxide to easily create composites with various materials, and also be used as coating films and functional films.
Recently there have been studies on developing graphene oxide for potential use in various functional materials such as catalysts and in next-generation battery materials, antibacterial and antiviral materials, coatings, lubricants, and water purification. As graphene oxide has a high aspect ratio and high dispersibility, it can form a network in the host material with a small amount added, and exhibits various functions. Graphene oxide can also form in extremely thin films by itself, so it can be used in membrane and film shapes as well.
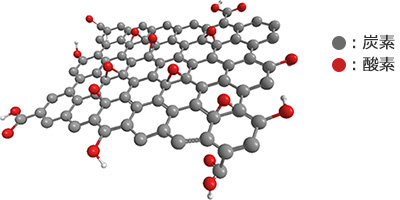
Graphene oxide is synthesized via an oxidation reaction of graphite. However, this synthesis uses a powerful oxidizing agent in a strong acid solvent. This generally makes the mass production of graphene oxide difficult, and its usage limited to research purposes in minimal amounts that are sold at an extremely high price. The development of graphene oxide for industrial use has been lagging, and so its excellent properties have yet to be commercialized. In that context, Nippon Shokubai has resolve the various issues related to the oxidation reaction, and successfully mass produced samples of graphene oxide that.
Nippon Shokubai’s graphene oxide can create composites and exhibit functions using its high dispersibility for various applications unlike other nanocarbon materials. We can customize our graphene oxide to suit the user’s requirements and preferences.
Examples of Applications
Please check the examples of applications.
Graphene Oxide and Other Nanocarbon Materials
Graphene Oxide
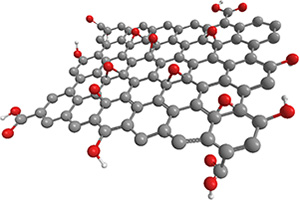
Shape: Sheet (two-dimensional material)
Graphene with oxygen functional groups.
Synthesis itself has been practiced for a long time, but mass production has been difficult.
We succeeded in mass production trial.
Graphene
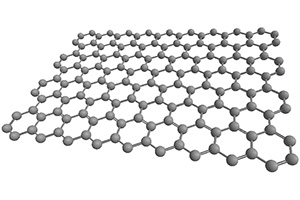
Shape: Sheet (two-dimensional material)
In 2004, Geim and Novoselov isolated it and won the 2010 Nobel Prize in Physics.
Carbon Nanotube(CNT)
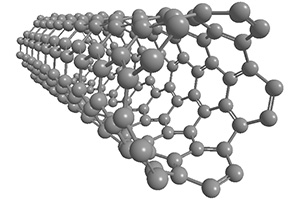
Shape:fiber(one-dimensional material)
Discovered in 1991 by Professor Iijima of Meijo University.
Divided into the following according to the number of layers
・Multilayer(MWCNT)
・Single layer(SWCNT)
Fullerene(C60)
Shape:sphere(zero-dimensional material)
Discovered by Kroto et al. in 1985 and won the Nobel Prize in Chemistry in 1996.
Graphene and graphene oxide have similar shapes, but actually, how they have developed is completely different.
Graphene is synthesized by chemical vapor deposition (CVD), it is possible to obtain single-layer film through a reaction of using methane, alcohol and other hydrocarbon gases as raw materials with metal catalysts. However, mass producing uniform and large area thin film is reportedly difficult and quite costly.
Multilayer graphene obtained by the mechanical exfoliation of graphite is called exfoliated graphene or graphene nano platelet (GNP). It is alsocalled graphene; but as it is not single layerso it is not graphene. This material also has low dispersibility and is difficult to handle.
In contrast, graphene oxide can be obtained by the liquid-phase oxidation of natural graphite (an aggregate of graphene), which is exfoliated after oxidation to a single layer. Unlike mechanical exfoliation, the oxidation method can produce single layers.
Graphene oxide has a sheet structure with oxygen-functional groups. It also has high dispersibility, which so far has been difficult to achieve in existing nanocarbon materials such as carbon nanotubes and graphene.
Nippon Shokubai is aiming to commercialize single-layer graphene oxide to realize mass production, based on the results of running pilot scale tests* to verify the liquid-phase oxidation process.
Refer to the news release “Nippon Shokubai succeeds in mass production test of graphene oxide-based materials.”
Precautions
- In principle, sample shipments are limited to within Japan.
- This is a product in development, so please consider separately matters such as conformance to the laws, regulations, and types of standards related to usage.
- The data listed in this page are examples, and are not guarantees of performance.
- Please note that the contents (and specifications) listed on this page may be changed without prior notice.
- The Company does not take any responsibility whatsoever for any issues that may arise in relation to the usage of this product, including, but not limited to, that its use will not infringe upon the intellectual property rights of any third party.