In development Intermediate Water Polymers
- Main Applications
Biocompatible polymers
- Applications
Basic Information
The intermediate water polymers developed by Nippon Shokubai are highly hydrophilic, with biocompatibility that has been confirmed in a joint study with Professor Masaru Tanaka of the Institute for Materials Chemistry and Engineering (IMCE) of Kyushu University.
What are Intermediate Water Polymers?
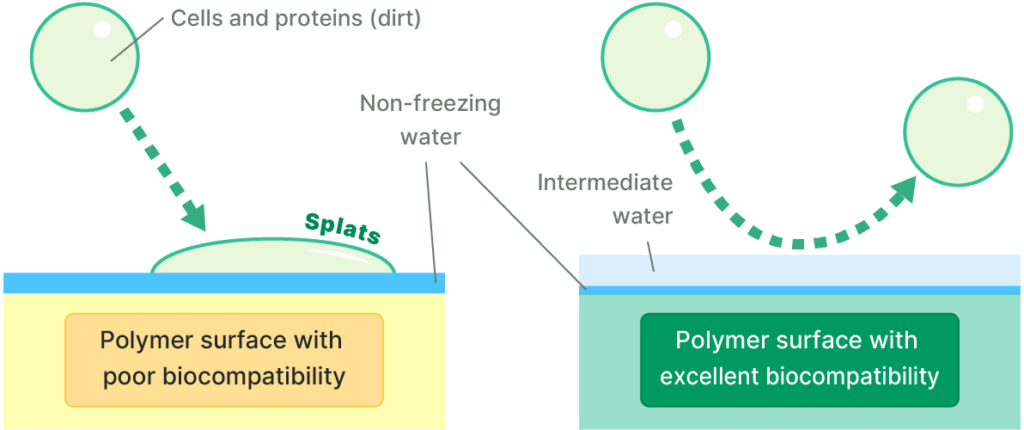
Professor Masaru Tanaka’s research revealed that polymer materials with excellent biocompatibility share a common feature of having intermediate water.*
Intermediate water polymers are polymer materials that have been designed and developed to include more intermediate water.

* Intermediate Water
When polymer materials are hydrated with water, the hydrated water takes on various states through its interaction with the polymers. These states are roughly grouped into three types depending on the degree of interaction: free water, intermediate water, and non-freezing water. Intermediate water is the middle state between free water and non-freezing water, and is water that gently interacts with polymers.
| Interaction with Polymers | Freezing Point | ||
|---|---|---|---|
| Free Water | Weak | 0℃ | Freezes and melts at 0°C, just like ordinary water. |
| Intermediate Water | Moderate | < 0°C | Gently interacts with polymer materials, and freezes and melts at temperatures under 0°C. |
| Non-freezing Water | Strong | Not detected | Does not freeze even at (minus) -150°C. |
Features and Properties
Has excellent biocompatibility.
Details of Functions
The in-vivo foreign substances reaction, rejection reaction, and other actions start when cells recognize foreign substances. Cells recognize the external world through proteins.
Intermediate water has a loose structure that enables it to remain on the surface of polymers for a long time, acting like a cushion layer that blocks contact between the surface of polymers and proteins and cells. This is why polymers with a high content of intermediate water are not considered by living organisms to be foreign substances.
In contrast, studies* have indicated that non-freezing water formed in polymers easily dehydrates proteins and causes protein denaturation, which is not preferable in terms of biocompatibility.
Nippon Shokubai’s intermediate water polymers contain an abundant amount of intermediate water and little non-freezing water, which give them stable biocompatibility.
* Journal of Biomaterials Science, Polymer Edition, Vol.3, p.127 (1992)
Related Products
PMEA, which is currently used widely as a biocompatible material, is known to also contain intermediate water. Nippon Shokubai has its product lineup in the methoxyethyl acrylate AME, which is a raw material of PMEA.